Determine Which of the Following Ions Is More Stable.
The donation of an electron is then 1. Use resonance to explain your answer.

Which Is More Stable Fe2 Or Fe3 Quora
As you can see N needs only 6 more electrons to have an octet so.
. Although Mn 3 ion has half-filled t 2g. Determine which of the following ions is more stable. Kr 5s 1 4d 7.
Ag 2. Determine the shape and point group of the following molecules and ions. Place the cation first in the formula followed by the anion.
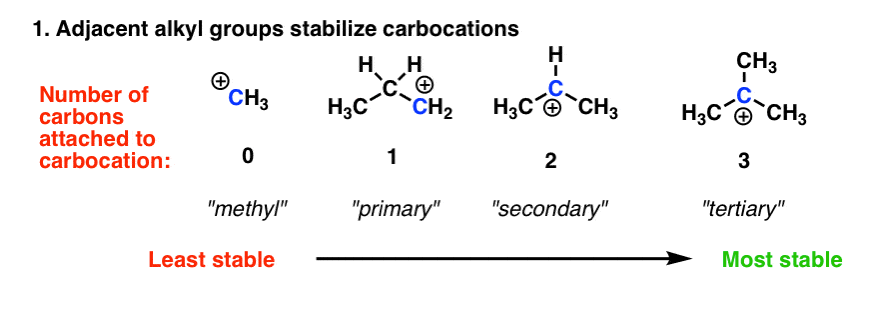
Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighbouring groups in hyperconjugation and resonance. 632 Write the formula including the charge for each of the following polyatomic ions. Variable charge is when a metal has two or more ion.
Among simple organic compounds the most stable molecular ions are those from aromatic rings other conjugated pi-electron systems and cycloalkanes. Since the bond skeleton consists of 4 electrons it follows that we have 6 electrons 10 4 leftover to distributeTo distribute the 6 electrons we start from the outer atoms but since H can have only 2 valence electrons and it does have its 2 valence electrons we move on to N in the bond skeleton a. Radicals are species with an unpaired electron.
Although Mn 2 is the most stable ion for manganese. Exam 1 page 6 Selected Character Tables. Orbitals is Chromium III ion.
The hybridization of a molecule is measured to determine the shape of the molecule. OCH -CH-CH OCH -CH - OCH Submit Hequest Answer. Hence among the given ions the only ion which occupies half-filled t 2g.
Stability to transition metal ion is directly proportional to the unpaired electron. Ag 1 vs. The mass spectrum of dodecane on the right illustrates the behavior of an unbranched alkane.
Tell whether the molecule or ion is polar or non-polar. The shape of a carbon dioxide molecule is linear. More energy is required to break the bonds between the ions in the compound and between the water molecules than is returned.
The more alkyl groups the more stable the carbocation. Larger bond order means greater bond strength. 1 o 2 o and 3 o stand for primary secondary and tertiary respectively.
Kr 5s 2 4d 6 vs. Alcohols ethers and highly branched alkanes generally show the greatest tendency toward fragmentation. An atom that accepts an electron to achieve a more stable configuration is assigned an oxidation number of -1.
Identify the formulas and charges of the cation and anion. Any time a system shifts from a more stable state to a less stable state the potential energy of the system increases. Determine if the following species are oxidized or reduced.
For the same reason putting the positive charge next to an electron-withdrawing group makes it less stable. We know that half-filled and fully filled orbitals are more stable than partially filled orbitals. Hence among the given ions the only ion which occupies half-filled t 2g.
Determine the more stable configuration between the following pair. Determine which of the following ions is more stable. This shows the number of carbons alkyl groups connected to the politely charged carbon.
But the charge of the nucleus increases from 8 on Oxygen to 13 for Aluminum. Orbital which reduces the stability. We know that half-filled and fully filled orbitals are more stable than another half nor fully filled orbitals.
OCH -CH-CH OCH -CH - OCH Submit Hequest Answer. Isoelectronic Ions- - ions containing the same number of electrons. Mn 3223d 44s 0t 2g.
Of the two ions which do you predict to be the more stable and very briefly why. The H2- ion is more stable than H2 since it has an additional electron to produce a net lowering of energy. Which of the following ions is more stable.
Within each group rank the anions from most stable to least stable. Orbitals is Chromium III ion. Cu2 e- rightarrow Cu.
So the same number of electrons is Aluminum Al 3 will be bound much closer to the nucleus than the. Determine how many of each ion type is needed to make a neutral compound. Which of the following ions is more stable.
1The concentration of sodium ions are highest in 2The concentration of potassium ions are highest in 3The concentration of phosphate ions are highest in 4The concentration of bicarbonate ions are highest in 5The concentration of protein anions are highest in. If more than one unit of a polyatomic ion is needed place parentheses around the polyatomic ion. The exactly half filled and completely filled d - orbitals are extra stableCr3 21 3d3 4s0 -3 unpaired electronsV3 20 3d2 4so -2 unpaired electronsTi3 19 3d1 4s0 -1 unpaired electronMn3 22 3d4 4so - 4 unpaired electronsSo Mn3 ion is most stable in aqueous solution.
Sometimes remote groups provide additional stabilization for a cation. Indicate whether each of the following anions would be more stable or less stable than a phenoxide anion and explain why. Mn 3223d 44s 0t 2g 4e g0.
The bond order for CN- is 2. For example O 2- F - Na Mg 2 and Al 3 all have the electron configuration of Neon. 62 State the number of electrons that must be gained by atoms of each of the following to achieve a stable electron configuration.

3 Factors That Stabilize Carbocations
Which Is More Stable Fe2 Or Fe3 Quora
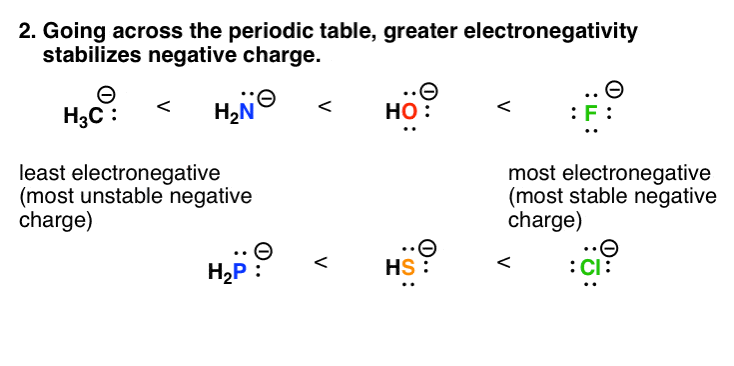
7 Factors That Stabilize Negative Charge In Organic Chemistry
No comments for "Determine Which of the Following Ions Is More Stable."
Post a Comment